Stéphane BACH
Scientific manager of the KISSf screening facility, working on protein kinase inhibitors, notably those characterized from various marine resources.
Co-founder of SeaBeLife Biotech (www.seabelife.com/en/), which develops novel therapies for the treatment of acute liver and kidney disorders.
I am a Senior Research Engineer in chemobiology with more than 25 years of research experience. I obtained my PhD in Agricultural Sciences and Bioengineering from the Faculty of Gembloux Agro-Bio Tech (Belgium) in 2000, and earned my Habilitation à Diriger des Recherches (HDR) in 2007 from the University of Western Brittany (Université de Bretagne Occidentale, Brest).
In 2001, I moved to the Roscoff Marine Station (France), where I developed various screening assays for the discovery and characterization of small-molecule inhibitors targeting disease-relevant protein kinases involved in cancer and neurodegenerative disorders.
I am currently the scientific manager of the Kinase Inhibitor Specialized Screening Facility (KISSf), which focuses on protein kinase inhibitors, notably those derived from marine resources. A second part of my work concerns the characterization of new generations of RIPK1 inhibitors with multi-target profiles. RIPK1 is a key regulator of a specialized form of programmed cell death known as necroptosis. I therefore have a particular interest in identifying and characterizing marine bioactive molecules that modulate necroptotic cell death for the development of new human therapeutics.
Linked to this activity, I am also the co-founder of SeaBeLife Biotech, a biotechnology company developing patented candidate molecules designed to block two forms of regulated cell death and thereby protect organs — notably the liver and eyes — from disease-related damage.
I am the author of more than 125 scientific publications and four patents.

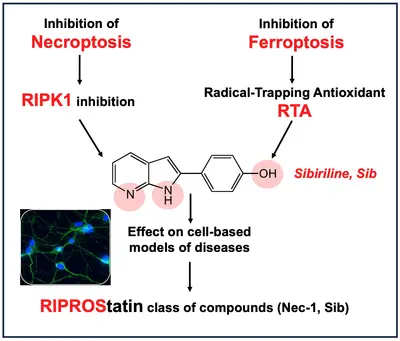
RIPROStatins a new class of drugs acting on multiple cell death pathways
Publications
126. Khelf-Maghraoui A., Bentabed-Ababsa G., Mast N., Erb W., Hurvois J-P., Roisnel T.J., Richy N., Calvez G., Mongin O., Piroud C., Robert T., Bach S. and Mongin F. 2025. BF2 Complexes of N-arylated 1-amino-anthraquinone, -xanthone, -thioxanthone, and Related Compounds: Synthesis and Properties. Eur. J. Org. Chem., 0, e202500470. https://doi.org/10.1002/ejoc.202500470.
125. Delehouzé, C., Mallais, M., Comte A., Lucas, R., Baratte, B., Bélal, S., Autret, A., Py, N., Steinschneider, R., Adoux, L., Saintpierre, B., Letourneur, F., Robert, T., Cougoule, C., Bomfim, C., Planès, R., Péricat, D., Bulinski, J., C., Dimanche-Boitrel, M.-T., Goekjian, P., Meunier, E., Rousselot, M., Pratt, D. A., Bach, S.*. 2025. Sibiriline, a novel dual inhibitor of necroptosis and ferroptosis, prevents RIPK1 kinase activity and (phospho)lipid peroxidation as a potential therapeutic strategy. Cell Death Discov. 11, 552. https://doi.org/10.1038/s41420-025-02852-8.
124. Singh, V. K., Justaud, F., Brahmaiah, D., Kumar, N. S., Baratte, B., Robert, T., Bach, S., Reddy, C. R., Levoin, N., Grée, R. L. 2025. Research towards selective inhibition of the CLK3 kinase. Beilstein J. Org. Chem. 2025, 21, 2250–2259. doi:10.3762/bjoc.21.172.
123. Tisseur L., Logé C., Cojean S., Gassama K., Karcher L., Pagniez F., Cavé C., Bernadat G., Loiseau P.M., Bach S., Thiéfaine J., Bonnet J., Picot C., Tomasoni C., Leclercq O., Baratte B., Robert T., Le Pape P., Rachidi N., Bazin MA. and Marchand P. 2025. Pharmacophore-guided optimization of the hit compound CTN1122 in the design of promising imidazo[1,2- a]pyrazine derivatives targeting the casein kinase 1 for antileishmanial therapy. RSC Med Chem. doi: 10.1039/d5md00257e.
122. Vinay Kumar Singh V. K., Justaud F., Brahmaiah D., Kumar N.S., Baratte B., Robert T., Bach S., Reddy C.R., Levoin N., Grée R. 2025. Research towards selective inhibition of the CLK3 kinase. Beilstein Arch. 2025, 202544. https://doi.org/10.3762/bxiv.2025.44.v1
121. de Lima J.R., da Silva Góes A.J., de Oliveira Borba E.F., da Silva M.A., Caiana R.R.A., Rodrigues M.D.D., de Lima Silva M.S., Chagas C.A., Baratte B., Robert T., Bach S., Ourliac-Garnier I., Marchand P. and da Silva T. 2025. New Insights into the Anticancer Effects and Toxicogenomic Safety of Two β-Lapachone Derivatives. Pharmaceuticals 2025, 18, x. https://doi.org/10.3390/xxxxx.
120. Janeczko M., Masłyk M., Demchuk O.M., Kurowska-Okoń A., Kwaśnik M., Górka K., Martyna A., Foll-Josselin B., Ruchaud S., Bach S., Woliński P., Jasiński R., Mirosław B., Sadczuk M. & Kubiński K. 2025. Development of a novel family of antifungal agents based on a quinone methide oxime framework. Sci Rep 15, 13458 (2025). https://doi.org/10.1038/s41598-025-98609-5
119. Ali Dridi M., Hasyeoui M., Lassagne F., Erb W., Piroud C., Robert T., Bach S., Samarat A., Touil S., Mongin F. 2025. On the Synthesis of [1,3]Azaphospholo[4,5-f]quino(xa)lines and 2,3-Dihydro-[1,3]azaphospholo[4,5-f]quino(xa)line 1-oxides. Eur. J. Org. Chem. e202500080.
118. Tisseur L., Cojean S., Gassama K., Logé C., Pagniez F., Cavé C., Bernadat G., Loiseau P. M., Bach S., Thiéfaine J., Picot C., Tomasoni C., Leclercq O., Baratte B., Robert T., Le Pape P., Rachidi N., Bazin M.-A. and Marchand P. 2025. Investigating the C2 Modulation of the Imidazo[1,2-]pyrazine-Based Hit Compound CTN1122: Synthesis, in vitro Antileishmanial Activity, Cytotoxicity and Casein Kinase 1 Inhibition. ChemMedChem, e202400862. https://doi.org/10.1002/cmdc.202400862.
117. Diakité A. S., Ambeu-Loko C. N. M., Yapi A. D., Logé C., Kacou A., Kra S., Baratte B., Bach S., Ruchaud S., Sissouma D., Ouattara M. and Robert J.-M. 2024. Design and Synthesis of Functionalized 2,4-Diamino-1,3,5-Triazines, Potential Inhibitors Involved in Immune and Inflammatory Response. International Journal of Pharmaceutical Research and Allied Sciences, 13(4), 1-11. https://doi.org/10.51847/hsT2C61XWx.
116. Broudic N., Pacheco- Benichou A., Corbière C., Baratte B., Robert T., Bach S., Solhi H., Le Guével R., Fruit C. and Besson T. 2024. Novel Thiazole-Fused [4,5-g] or [5,4-g]Quinazolin-8-ones and Their Quinazoline Analogues: Synthesis and Biological Evaluation. Pharmaceuticals 2024, 17, 1452. https://doi.org/10.3390/ph17111452.
115. Pham G.N., Josselin B., Cousseau A., Baratte B., Dayras M., Le Meur C., Debaets S., Weill A., Robert T., Burgaud G., Probert I., Abdoul-Latif F.M., Boyer L., Bach S.* and Mehiri M.* 2024. New Fusarochromanone Derivatives from the Marine Fungus Fusarium equiseti UBOCC-A-117302. Mar. Drugs 2024, 22,444. https://doi.org/10.3390/ md22100444.
114. Bakalakou V-A., Mavroidi B., Kalampaliki A.D., Josselin B., Bach S., Skaltsounis A.-L., Marakos P., Pouli N., Pelecanou M., Myrianthopoulos V., Ruchaud S. and Kostakis I.K. 2024. The pyrazolo[4,3-c]pyrazole core as a novel and versatile scaffold for developing dual DYRK1A-CLK1 inhibitors targeting key processes of Alzheimer’s disease pathology. European Journal of Medicinal Chemistry Reports. 100193. ISSN 2772-4174, https://doi.org/10.1016/j.ejmcr.2024.100193.
113. Eugenio M.S., Imerzoukene G., Hamon A., Piquet-Pellorce C., Rapin A., Delehouzé, C., Belal S., Autret A., Akakpo J., Jaeschke H., Bach S., Samson M., Le Seyec J., Dimanche-Boitrel M.-T. and Rousselot M. 2024. FRI-333 Sibiriline, an inhibitor of regulated necrosis, protects mice against Acetaminophen-induced liver injury, alone or in combination with N-acetyl-cysteine. Journal of Hepatology. Volume 80, Supplement 1,Pages S109-S110, ISSN 0168-8278. https://doi.org/10.1016/S0168-8278(24)00632-9.
112. Laure A., Royet C., Bihel F., Baratte B., Bach S., Peyressatre M. and Morris M. C. 2024. Ethaverine and Papaverine target cyclin-dependent kinase 5 and inhibit lung cancer cell proliferation and migration. ACS Pharmacology & Translational Science. Manuscript No.: pt-2024-00023e (acsptsci.4c00023). doi.org/10.1021/acsptsci.4c00023.
111. Kieffer C., Primas N., Hutter S., Merckx A., Reininger L., Bach S., Ruchaud S., Gaillard F., Laget M., Amrane D., Hervé L., Castera-Ducros C., Renault J., Dumètre A., Rault S., Doerig C., Rathelot P., Vanelle P., Azas N. and Verhaeghe P. 2024. Target fishing reveals PfPYK-1 and PfRab6 as potential targets of an antiplasmodial 4-anilino-2-trichloromethylquinazoline hit compound. Bioorg Med Chem. Mar 15;102:117654. doi: 10.1016/j.bmc.2024.117654. Epub 2024 Feb 28. PMID: 38452406
110. Broudic N., Pacheco-Benichou A., Robert T., Bach S., Solhi H., Le Guevel R., Fruit C. and Besson T. 2023. Synthesis and biological evaluation of linear thiazolo[4,5-g] and [5,4-g]quinazolines, analogues of V-shaped DYRK1A inhibitors EHT1610 and FC162., in Proceedings of the 9th International Electronic Conference on Medicinal Chemistry, 1–30 November 2023, MDPI: Basel, Switzerland, doi:10.3390/ECMC2023-15566
109. Blouet C., Letast S., Robert T., Bach S., Pinaud N., Joubert N., Viaud-Massuard M-C., Guillon J., Logé C. and Denevault-Sabourin C. 2023. Potassium 6-Oxo-7,13,16,22-tetraazatetracyclo[12.6.2.18,12.017,21]tricosa-1(20),8(23),9,11,14,16,18,21-octaen-2-yne-15-carboxylate. Molbank. 2023; 2023(4):M1735. https://doi.org/10.3390/M1735.
108. Moradi M., Mousavi A., Emamgholipour Z., Giovannini J., Moghimi S., Peytam F., Honarmand A., Bach S.* and Foroumadi A.* 2023. Quinazoline-based VEGFR-2 inhibitors as potential anti-angiogenic agents: A contemporary perspective of SAR and molecular docking studies. European Journal of Medicinal Chemistry, Vol 259, 115626, https://doi.org/10.1016/j.ejmech.2023.115626.
107. Ribeiro E. Silva A., Diallo M.-A., Sausset A., Robert T., Bach S., Bussière F.I., Laurent F., Lacroix-Lamandé S. and Silvestre A. 2023. Overexpression of Eimeria tenella Rhoptry Kinase 2 Induces Early Production of Schizonts. Microbiology Spectrum. DOI: https://doi.org/10.1128/spectrum.00137-23.
106. Jabeur R., Corbel C., Loyer P., Le Parc A., Le Grand A., Comte A., Bach S., André-Leroux G., Sire O., Ben Mansour H. and Le Tilly V. 2023. Identification of Novel Compounds Inhibiting the Kinase Activity of the CDK5/p25 Complex via Direct Binding to p25. Biochemistry. Apr 19. doi: 10.1021/acs.biochem.2c00691. Online ahead of print. PMID: 37074084
105. Cormier P., Boutet A., Colas P., Ruchaud S., Bach S., Thomas W., Egée S., Morales J. and Roch F. 2023. The Roscoff Biological Station in the era of cellular and molecular biology: Focus on the 50 last years of research on marine animals. Cah. Biol. Mar. (2023) 64: 35-52. DOI: 10.21411/CBM.A.C705BF49
104. M. Hasyeoui M., Lassagne F., Erb W., Nael M., Elokely K.M., Chaikuad A., Knapp S., Jorda A., Vallés S.L., Quissac E., Verreault M., Robert T., Bach S., Samarat A., Mongin F. 2023. Oxazolo[5,4–f]quinoxaline-type selective inhibitors of glycogen synthase kinase-3α (GSK-3α): Development and impact on temozolomide treatment of glioblastoma cells, Bioorganic Chemistry (2023), doi: https://doi.org/10.1016/j.bioorg.2023.106456
103. Hasanvand Z., Bakhshaiesh T.O., Peytam F., Firoozpour L., Hosseinzadeh E., Motahari R., Moghimi S., Nazeri E., Toolabi M., Momeni F., Bijanzadeh H., Khalaj A., Baratte B., Josselin B., Robert T., Bach S., Esmaeili R., Foroumadi A. 2023. Imidazo[1,2-a]quinazolines as novel, potent EGFR-TK inhibitors: design, synthesis, bioactivity evaluation, and in silico studies. Bioorganic Chemistry. https://doi.org/10.1016/j.bioorg.2023.106383
102. Mast N., Erb W., Nauton L., Moreau P., Mongin O., Roisnel T., Macaigne M., Robert T., Bach S., Picot L., Thiéry V., J.-P. Hurvois and Mongin F. 2023. From benzofuro-, benzothieno- and 10-methylindolo-[2,3-b]-fused benzothiopyrano[4,3,2-de]quinolines to the corresponding benzothiopyrano[4,3,2-de]1,8-naphthyridines: synthesis and properties of these hexacyclic heteroaromatic compounds. New J. Chem., 2023, 47, 258. https://doi.org/10.1039/d2nj04567b
101. Dhiabi M., Bouattour A., Fakhfakh M., Abid S., Paquin L., Robert T., Bach S., Bazureau J-P. and Ammar H. 2023. Practical approach to N-benzyl derivatives of 2-amino-8-methoxy-4H-chromene-3-carbonitrile by reductive amination: Exploration of their effects against protein kinases and in silico ADME profiling. Journal of Molecular Structure. Vol. 1274, 15 February 2023, https://doi.org/10.1016/j.molstruc.2022.134319.
100. Caputo A., Sartini S., Levati E., Minato I., Elisi G.M., Di Stasi A., Guillou C., Goekjian P.G., Garcia P., Gueyrard D., Bach S., Comte, A.; Ottonello, S., Rivara, S. and Montanini, B. 2022. An Optimized Workflow for the Discovery of New Antimicrobial Compounds Targeting Bacterial RNA Polymerase Complex Formation. Antibiotics, 11, 1449. https://doi.org/10.3390/antibiotics11101449.
99. Brahmaiah D., Bhavani A.K.D., Aparna P., Kumar N.S., Solhi H., Le Guevel R., Baratte B., Robert T., Ruchaud S., Bach S., Jadav S.S., Reddy C.R., Mosset P., Gouault N., Levoin N. and Grée R. 2022. Structure Activity Relationship Studies around DB18, a Potent and Selective Inhibitor of CLK Kinases. Molecules. 2022 Sep 20;27(19):6149. doi: 10.3390/molecules27196149.
98. Avula S., Peng X., Lang X., Tortorella M., Josselin B., Bach S., Bourg S., Bonnet P., Buron F., Ruchaud S., Routier S. and Neagoie C. 2022. Design and biological evaluation of substituted 5,7-dihydro-6H-indolo[2,3-c]quinolin-6-one as novel selective Haspin inhibitors. J Enzyme Inhib Med Chem. 2022 Dec;37(1):1632-1650. doi: 10.1080/14756366.2022.2082419.
97. Delehouzé C., Comte A., Leon-Icaza S.A., Cougoule C., Hauteville M., Goekjian P., Bulinski J.C., Dimanche-Boitrel M.-T., Meunier E., Rousselot M., Bach S. 2022. Nigratine as dual inhibitor of necroptosis and ferroptosis regulated cell death. Scientific Reports 12, 5118. https://doi.org/10.1038/s41598-022-09019-w
96. Almeida et al. The Franco-Brazilian network on natural products (FB2NP): a new network promoting cooperation and exchanges in natural products research. 2022. Quim. Nova, Vol. 45, No. 1, 1-3, 2022 http://dx.doi.org/10.21577/0100-4042.20170847 .
95. Dao V.H., Ourliac-Garnier I., Logé C., McCarthy F.O., Bach S., da Silva T.G., Denevault-Sabourin C., Thiéfaine J., Baratte B., Robert T., Gouilleux F., Brachet-Botineau M., Bazin M.-A., Marchand P. 2021. Dibenzofuran Derivatives Inspired from Cercosporamide as Dual Inhibitors of Pim and CLK1 Kinases. Molecules, 26, 6572. https://doi.org/10.3390/molecules26216572.
94. Choudhary B. S., Sukanya, Mehta P., Bach S., Ruchaud S., Robert T., Josselin B., Filipek S., Malik R. 2021. Discovery of thiazolidin-4-one analogue as selective GSK-3β inhibitor through structure based virtual screening. Bioorganic & Medicinal Chemistry Letters. Volume 52 (128375). https://doi.org/10.1016/j.bmcl.2021.128375.
93. Pieterse L., Beteck R.M., Baratte B., Jesumorotia O., Robert T., Ruchaud S., Bach S., Legoabe L. J. 2021. Synthesis and biological evaluation of selected 7H-pyrrolo[2,3-d]pyrimidine derivatives as novel CDK9/CyclinT and Haspin inhibitors. Chemico-Biological Interactions. https://doi.org/10.1016/j.cbi.2021.109643.
92. Ahmadu, A.A., Delehouzé, C., Haruna, A., Mustapha, L., Lawal, B.A., Udobre, A., Baratte, B., Triscornia, C., Autret, A., Robert, T., Bulinski, J.C., Rousselot, M., Simoes Eugénio, M., Dimanche-Boitrel, M., Petzer, J.P., Legoabe, L.J., Bach, S. 2021. Betulin, a Newly Characterized Compound in Acacia Auriculiformis Bark, is a Multi-target Protein Kinase Inhibitor. Molecules 2021, 26, 4599. https://doi.org/10.3390/molecules26154599. This article was previously published as preprint. Preprints 2021, 2021060115 (doi: 10.20944/preprints202106.0115.v1).
91. Qhobosheane M., Beteck R. M., Baratte B., Robert T., Ruchaud S., Bach S., Legoabe L.J. 2021. Exploration of 7-azaindole-coumaranone hybrids and their analogues as protein kinase inhibitors. Chemico-Biological Interactions, Available online 25 April 2021, 109478. https://doi.org/10.1016/j.cbi.2021.109478
90. Lassagne F.*, Sims J.M., Erb W., Mongin O., Richy N., El Osmani N., Fajloun Z.*, Picot L., Thiery V., Robert T., Bach S.*, Dorcet V., Roisnel T. and Mongin F.* 2021. Thiazolo[5,4‐f]quinoxalines, oxazolo[5,4‐f]quinoxalines and pyrazino[b,e]isatins: synthesis from 6‐aminoquinoxalines and properties. Eur. J. Org. Chem. https://doi.org/10.1002/ejoc.202100362
89. Oyallon B., Brachet-Botineau M., Logé C., Robert T., Bach S., Ibrahim S., Raoul W., Croix C., Berthelot P., Guillon J., Pinaud N., Gouilleux F., Viaud-Massuard M-C., Denevault-Sabourin C. 2021. New Quinoxaline Derivatives as Dual Pim-1/2 Kinase Inhibitors: Design, Synthesis and Biological Evaluation. Molecules. 26(4):867. https://doi.org/10.3390/molecules26040867
88. Juillet C., Ermolenko L., Boyarskaya D., Baratte B., Josselin B., Nedev H., Bach S., Iorga B., Bignon J., Ruchaud S., Al-Mourabit A. 2021. From Synthetic Simplified Marine Metabolite Analogues to New Selective Allosteric Inhibitor of Aurora B Kinase. Journal of Medicinal Chemistry. 64(2), 1197-1219. https://doi.org/10.1021/acs.jmedchem.0c02064
87. Brahmaiah D., Bhavani A., Aparna P., Kumar N., Solhi H., Le Guevel R., Baratte B., Ruchaud S., Bach S., Jadav S., Reddy C., Roisnel T., Mosset P., Levoin N. and Grée R. 2021. Discovery of DB18, a potent inhibitor of CLK kinases with a high selectivity against DYRK1A kinase. Bioorganic & Medicinal Chemistry. DOI: https://doi.org/10.1016/j.bmc.2020.115962
86. Delehouzé C., Comte A., Hauteville M., Goekjian P., Dimanche-Boitrel M-T., Rousselot M.* and Bach S.* 2020. Nigratine as first-in-class dual inhibitor of necroptosis and ferroptosis regulated cell death. Preprint. BioRxiv 2020.12.15.422885; doi: https://doi.org/10.1101/2020.12.15.422885.
85. Elie J., Feizbakhsh O., Desban N., Josselin B., Baratte B., Bescond A., Duez J., Fant X., Bach S., Marie D., Place M., Ben Salah S., Chartier A., Berteina-Raboin S., Chaikuad A., Knapp S., Carles F., Bonnet P., Buron F., Routier S. and Ruchaud S. 2020. Design of new disubstituted imidazo[1,2-b]pyridazine derivatives as selective Haspin inhibitors. Synthesis, binding mode and anticancer biological evaluation, Journal of Enzyme Inhibition and Medicinal Chemistry, 35:1, 1840-1853, DOI: 10.1080/14756366.2020.1825408.
84. Wilde M., Arzur D., Baratte B., Lefebvre D., Robert T., Roisnel T., Le Jossic-Corcos C., Bach S.*, Corcos L.* and Erb W.* 2020. Regorafenib analogues and their ferrocenic counterparts: synthesis and biological evaluation. The article was first published on 10 Nov 2020. New J. Chem., https://doi.org/10.1039/D0NJ05334A.
83. Princival I., Princival J., da Silva E., Lima S., Carregos J., de Lucenaa A., Halwass F., Franc J., Ferreir L., Hernandes M., Saraiv K., Peixoto K., Baratte B., Robert T., Bach S., Gomes D., Paiva D., Marchand P., Rodrigues M. and da Silva T. 2021. Streptomyces hygroscopicus UFPEDA 3370: a valuable source of the potent cytotoxic agent nigericin and its evaluation against human colorectal cancer cells. Chemico-Biological Interactions. 333:109316. doi: 10.1016/j.cbi.2020.109316.
82. Bazin A., Cojean S., Pagniez F., Bernadat G., Cavé C., Ourliac-Garnier I., Nourrisson M.-R., Morgado C., Picot C., Leclercq O., Baratte B., Robert T., Späth G.F., Rachidi N., Bach S., Loiseau P.M., Le Pape P. and Marchand P. 2021. In vitro identification of imidazo[1,2-a]pyrazine-based antileishmanial agents and evaluation of L. major casein kinase 1 inhibition. European Journal of Medicinal Chemistry. Available online 23 October 2020, 112956. https://doi.org/10.1016/j.ejmech.2020.112956.
81. Tuescher J. M., Tailfeathers D., Kernéis S.M., Baratte B., Ruchaud S., Bach S., Pouny I., Sautel F., and Golsteyn R.M. 2020. The Canadian prairie plant Thermopsis rhombifolia contains luteolin, a flavone that inhibits Cyclin dependent kinase 9 and arrest cells in the G1-phase of the cell cycle. The Journal of Natural Health Product Research. Vol.2, n°2. https://doi.org/10.33211/jnhpr.12.
80. Esvan Y., Josselin B., Baratte B., Bach S., Ruchaud S., Anizon F., Giraud F. and Moreau P. 2020. Synthesis and kinase inhibitory potencies of new pyrido[3,4-g]quinazolines substituted at the 8-position. Arkivoc. https://doi.org/10.24820/ark.5550190.p011.268.
79. Cousseau A., Siano R., Probert I., Bach S. and Mehiri M. 2020. Marine Dinoflagellates as source of new bioactive structures. Studies in Natural Products Chemistry (Bioactive Natural Products), Vol 65 (1st Edition), ISBN 9780128179055. ELSEVIER SCIENCE PUBLISHERS – AMSTERDAM. Vol. 65, 125-171. https://doi.org/10.1016/B978-0-12-817905-5.00004-4.
78. Pieterse L., Legoabe L., Beteck R., Josselin B., Bach S. and Ruchaud S. 2020. Synthesis and biological evaluation of selected 7-azaindole derivatives as CDK9/Cyclin T and Haspin inhibitors. Medicinal Chemistry Research. Accepted: 7 May 2020. In press. doi.org/10.1007/s00044-020-02560-1.
77. Bouattour A., Fakhfakh M., Abid S., Paquin L., Le Guével R., Charlier T., Ruchaud S., Bach S., Bazureau J.-P. and Ammar H. 2020 Synthesis of new 2-phenylamino-4H-chromene-3-carbonitrile derivatives and their effects on tumor cell lines and against protein kinases. Internat. J. Org. Chem. 10, 88-103. doi: 10.4236/ijoc.2020.102006.
76. Bach S., Colas P. et Blondel M. 2020. La levure modèle et outil… aussi pour la recherche thérapeutique. Médecine & Sciences. m/s n° 5, vol. 36, 504-513, doi.org/10.1051/medsci/2020077.
75. Ibrahim N., Bonnet P., Briona J.D., Peyrata J.F., Bignonc J., Levaiquec H., Josselin B., Robert T., Colas P., Bach S., Messaoudia S., Alamia M., Hamze A. 2020. Identification of a new series of Flavopiridol-like structures as kinase inhibitors with high cytotoxic potency. European Journal of Medicinal Chemistry. Volume 199, 1 August 2020, 112355, doi.org/10.1016/j.ejmech.2020.112355.
74. Benchekroun M., Ermolenko L., Tran M.Q.,Vagneux A., Nedev H., Delehouzé C., Souab M., Baratte B., Josselin B., Iorga B., Ruchaud S., Bach S.* and Al-Mourabit A.* 2020. Discovery of Simplified Benzazole Fragments Derived From The Marine Benzosceptrin B as Necroptosis Inhibitors Involving The Receptor Interacting Protein Kinase-1. European Journal of Medicinal Chemistry. 201:112337. DOI: 10.1016/j.ejmech.2020.112337.
73. Qhobosheane MA., Legoabe LJ., Josselin B., Bach S., Ruchaud S., Petzer J., Beteck R. 2020. Synthesis and evaluation of 7-azaindole derivatives bearing benzocycloalkanone motifs as protein kinase inhibitors [published online ahead of print, 2020 Mar 31]. Bioorg Med Chem. 2020;115468. doi:10.1016/j.bmc.2020.115468.
72. Robert T., Johnson J.L., Guichaoua R., Yaron T.M., Bach S., Cantley L.C. and Colas P. 2020. Development of a CDK10/CycM in vitro Kinase Screening Assay and Identification of First Small-Molecule Inhibitors. Front. Chem., 27 February 2020. DOI: 10.3389/fchem.2020.00147.
71. Lassagne F., Duguépéroux C., Roca C., Pérez C., Martinez A., Baratte B., Robert T., Ruchaud S., Bach S., Erb W., Roisnel T. and Mongin F. 2019. From simple quinoxalines to potent oxazolo[5,4-f]quinoxaline inhibitors of glycogen-synthase kinase 3 (GSK3). 2019. Org. Biomol. Chem., DOI: 10.1039/C9OB02002K.
70. Nascimento da Cruz A.C., Brondani D.J., I´talo de Santana T., Oliveira da Silva L., da Oliveira Borba E.F., de Faria A.R., Ferreira Cavalcanti de Albuquerque J., Piessard S., Matos Ximenes R., Baratte B., Bach S., Ruchaud S., Bezerra Mendonça Junior F.J., Bazin M.-A., Montenegro Rabello M., Hernandes M.Z., Marchand P. and Gonçalves da Silva T. 2019. Biological Evaluation of Arylsemicarbazone Derivatives as Potential Anticancer Agents. Pharmaceuticals, 12, 169.
69. Mokhtari Brikci-Nigassa N., Nauton L., Moreau P., Mongin O., Duval R., Picot L., Thiéry V., Souab M., Ruchaud S., Bach S., Guevel R., Bentabed-Ababsa G., Erb W., Roisnel T., Dorcet V., Mongin F. 2019. Functionalization of 9-thioxanthone at the 1-position: from arylamino derivatives to [1]benzo(thio)pyrano[4,3,2-de]benzothieno[2,3-b]quinolines of biological interest, Bioorganic Chemistry, 103347, doi.org/10.1016/j.bioorg.2019.103347.
68. Nguyen TN., Feizbakhsh O., Sfecci E., Baratte B., Delehouzé C., Garcia A., Moulin C., Colas P., Ruchaud S.*, Mehiri M.*, and Bach S. * 2019. Kinase-Based Screening of Marine Natural Extracts Leads to the Identification of a Cytotoxic High Molecular Weight Metabolite from the Mediterranean Sponge Crambe tailliezi. Mar Drugs. 2019 Oct 9;17(10). pii: E569. doi: 10.3390/md17100569.
67. Ziane S., Mazari M.M., Safer A.M., Sad El Hachemi Amar A., Ruchaud S., Baratte B. and Bach S. 2019. Comparison between Conventional and Nonconventional Methods for the Synthesis of Some 2-Oxazolidinone Derivatives and Preliminary Investigation of Their Inhibitory Activity Against Certain Protein Kinases. Russian Journal of Organic Chemistry. July 2019, Volume 55, Issue 7 (1061–1069). doi.org/10.1134/S1070428019070248.
66. Sartini S, Levati E., Maccesi M., Guerra M., Spadoni G., Bach S., Benincasa M., Scocchi M., Ottonello S., Rivara S. and Montanini B. 2019. New Antimicrobials Targeting Bacterial RNA Polymerase Holoenzyme Assembly Identified with an in Vivo BRET-Based Discovery Platform. ACS Chem Biol. 2019 Jul 30. doi: 10.1021/acschembio.9b00178.
65. Khaldoun K., Safer A., Boukabcha N., Dege N., Ruchaud S., Souab M., Bach S., Chouaih A. and Saidi-Besbes S. 2019. Synthesis and evaluation of new isatin-aminorhodanine hybrids as PIM1 and CLK1 kinase inhibitors. Journal of Molecular Structure. 1192 (82-90). ISSN 0022-2860. doi.org/10.1016/j.molstruc.2019.04.122.
64. Zeinyeh W., Esvan Y. J., Josselin B., Baratte B., Bach S., Nauton L., Théry V., Ruchaud S., Anizon F., Giraud F. and Moreau P. 2019. Kinase inhibitions in pyrido[4,3-h] and [3,4-g]quinazolines: Synthesis, SAR and molecular modeling studies. Bioorganic & Medicinal Chemistry. ISSN 0968-0896. doi.org/10.1016/j.bmc.2019.04.005.
63. Oukoloff K., Coquelle N., Bartolini M., Naldi M., Le Guevel R., Bach S., Josselin B., Ruchaud S., Catto M., Pisani L., Denora N., Iacobazzi R.M., Silman I., Sussman J.L., Buron F., Colletier J.-P., Jean L., Routier S. and Renard P.-Y. 2019. Design, biological evaluation and X-ray crystallography of nanomolar multifunctional ligands targeting simultaneously acetylcholinesterase and glycogen synthase kinase-3. European Journal of Medicinal Chemistry, doi.org/10.1016/j.ejmech.2018.12.063.
62. Motuhi S.-E., Feizbakhsh O., Foll-Josselin B., Baratte B., Delehouzé C., Cousseau A., Fant X., Bulinski J.C., Payri C.E., Ruchaud S., Mehiri M.* and Bach S.* 2019. Neurymenolide A, a Novel Mitotic Spindle Poison from the New Caledonian Rhodophyta Phacelocarpus neurymenioides. Mar. Drugs, 17, 93. doi:10.3390/md17020093.
61. Tazarki H., Zeinyeha W., Esvana, Y.J., Snapp K., Chatterjee D., Schröder M., Joerger A. C., Khiarib J., Josselin B., Baratte B., Bach S., Ruchaud S., Anizona F., Giraud F. and Moreau P. 2019. New pyrido[3,4-g]quinazoline derivatives as CLK1 and DYRK1A inhibitors: synthesis, biological evaluation and binding mode analysis. European Journal of Medicinal Chemistry, https://doi.org/10.1016/j.ejmech.2019.01.052.
60. Masłyk M., Janeczko M., Martyna A., Czernik S., Tokarska-Rodak M., Chwedczuk M., Foll-Josselin B., Ruchaud S., Bach S., Demchuk O.M. and Kubiński K. 2019. The Anti-Candida albicans Agent 4-AN Inhibits Multiple Protein Kinases. Molecules, 24, 153. doi.org/10.3390/molecules24010153.
59. Lassagne F., Langlais T., Caytan E., Limanton E., Paquin L.*, Boullard M., Courtel C., Curbet I., Gédéon C., Lebreton J., Picot L.*, Thiery V., Souab M., Baratte B., Ruchaud S., Bach S.*, Roisnel T. and Mongin F. 2018. From quinoxaline, pyrido[2,3-b]pyrazine and pyrido[3,4-b]pyrazine to pyrazino-fused carbazoles and carbolines. Molecules. doi: 10.3390/molecules23112961.
58. Serive B. and Bach S. 2018. Marine Pigment Diversity: Applications and Potential. Chapter of the textbook «Blue Technologies: production and uses of marine molecules in a changing world». Edited by Stéphane La Barre and Stephen Bates, Wiley-Blackwell. ISBN: 978-3-527-34138-2.
57. Winfield H.J., Cahill M.M., O'Shea K.D., Pierce L.T., Robert T., Ruchaud S., Bach S., Marchand P. and McCarthy FO. 2018. Synthesis and anticancer activity of novel bisindolylhydroxymaleimide derivatives with potent GSK-3 kinase inhibition. Bioorg Med Chem. 2018 Aug 7;26(14):4209-4224. doi: 10.1016/j.bmc.2018.07.012. Epub 2018 Jul 9.
56. Dao V.H., Ourliac-Garnier I., Bazin M.-A., Jacquot C., Baratte B., Ruchaud S., Bach S., Grovel O., Le Pape P. and Marchand P. 2018. Benzofuro[3,2-d]pyrimidines inspired from cercosporamide CaPkc1 inhibitor: synthesis and evaluation of fluconazole susceptibility restoration. Bioorganic & Medicinal Chemistry Letters. BMCL-D-18-00505. DOI:10.1016/j.bmcl.2018.05.044.
55. Oyallon B., Brachet-Botineau M., Logé C., Bonnet P., Souab M., Robert T., Ruchaud S., Bach S., Berthelot P., Gouilleux F., Viaud-Massuard M.-C. and Denevault-Sabourin C. 2018. Structure-based design of novel quinoxaline-2-carboxylic acids and analogues as Pim-1 inhibitors. Eur. J. Med. Chem. 154:101-109. doi: 10.1016/j.ejmech.2018.04.056.
54. von Mässenhausen A., Tonnus W., Himmerkus N., Parmentier S., Saleh D., Rodriquez D., Ousingsawat J., Ang R.L., Weinberg J.M., Sanz A.B., Ortiz A., Zierleyn A., Becker J.U., Baratte B., Desban N., Bach S., Schiessl I.M., Nogusa S., Balachandran S., Anders H.J., Ting A.T., Bleich M., Degterev A., Kunzelmann K., Bornstein S.R., Green D.R., Hugo C. and Linkermann A. 2018. Phenytoin inhibits necroptosis. Cell Death Dis., 9(3):359. doi: 10.1038/s41419-018-0394-3.
53. Brikci-Nigassa N.M., Bentabed-Ababsa G.*, Erb W., Chevallier F., Picot L.*, Vitek L., Fleury A., Thiéry V., Souab M., Robert T., Ruchaud S., Bach S.*, Roisnel T. and Mongin F.*. 2018. 2-Aminophenones, a common precursor to N-aryl isatins and acridines endowed with bioactivities. Tetrahedron (TET-D-18-00014R1). DOI information: 10.1016/j.tet.2018.02.038.
52. Hamza-Reguig S., Bentabed-Ababsa G.*, Domingo L.R., Ríos-Gutiérrez M., Philippot S., Fontanay S., Duval R. E.*, Ruchaud S., Bach S.*, Roisnel T.* and Mongin F.* 2017. A combined experimental and theoretical study of the thermal [3+2] cycloaddition of carbonyl ylides with activated alkenes. J. Mol. Struct. doi.org/10.1016/j.molstruc.2017.12.052.
51. Ahmadu A. A., Agunu A., Nguyen T-N-D., Baratte B., Foll-Josselin B., Ruchaud S., Serive B. and Bach S. 2017. Constituents of Acacia nilotica Delile with Novel Kinase inhibitory activity. Planta Medica International Open. 4: e108–e113.
50. Bazureau J-P., Bouattour A., Fakhfakh M., Abid S., Ammar H., Paquin L., Le Guével R., Corlu, A., Ruchaud S. and Bach S. 2017. Reactivity under Microwave Irradiation of 2-Amino 4H-chromene-3-carbonitrile as Tool for the Construction of Potential Bioactive Derivatives. In Proceedings of the 3rd Int. Electron. Conf. Med. Chem., 1–30 November 2017; Sciforum Electronic Conference Series, Vol. 3, 2017 ; doi:10.3390/ecmc-3-04709.
49. Delehouzé C., Leverrier-Penna S., Le Cann F., Comte A., Jacquard-Fevai M., Delalande O., Desban N., Baratte B., Gallais I., Faurez F., Bonnet MC., Hauteville M., Goekjian PG., Thuillier, R., Favreau F., Vandenabeele P., Hauet T., Dimanche-Boitrel MT*., and Bach S*. 2017. 6E11, a new highly selective inhibitor of Receptor-Interacting Protein Kinase 1, protects cells against cold hypoxia-reoxygenation injury. Sci. Rep.; 7(1):12931. doi: 10.1038/s41598-017-12788-4.
48. Hedidi M., Maillard J., Erb W., Lassagne F., Halauko Y. S., Ivashkevich O. A., Matulis V. E., Roisnel T., Dorcet V., Hamze M., Fajloun Z., Baratte B., Ruchaud S., Bach S.* and Bentabed-Ababsa G.* and Mongin F.* 2017. Fused systems based on 2-aminopyrimidines: synthesis combining deprotolithiation-in situ zincation with N-arylation reactions and biological properties. Eur. J. Org. Chem. 10.1002/ejoc.201701004.
47. Kim J., Brown C.M., Kim M. K., Burrows E. H., Bach S., Lun D. S., Falkowski P. G. 2017. The effect of cell cycle arrest on intermediate metabolism in the marine diatom Phaeodactylum tricornutum. Proc Natl Acad Sci U S A. DOI: 10.1073/pnas.1711642114.
46. Le Cann F., Delehouzé C., Penna-Leverrier S., Filliol A., Comte A., Delalande O., Desban N., Baratte B., Gallais I., Piquet-Pellorce C., Faurez F., Bonnet M., Mettey Y., Goekjian P., Samson M., Vandenabeele P., Bach S.* and Dimanche-Boitrel M-T.* 2017. Sibiriline, a new small chemical inhibitor of Receptor-Interacting Protein Kinase 1, prevents immune-dependent hepatitis. FEBS Journal, 284(18):3050-3068, DOI: 10.1111/febs.14176.
45. Ali Bouattour A., Fakhfakh M., Abid S., Paquin L., Le Guével R., Corlu A., Ruchaud S., Bach S., Ammar H. and Bazureau J.-P. 2017. Microwave-assisted practical synthesis of 4-imino-3-phenyl-3,4-dihydro-1Hchromeno[2,3-d]pyrimidine-2(5H)-thione derivatives and exploration of their biological activities. Arkivoc 2017, part iv, 291-302.
44. Navarri M., Bondon A., Pottier S., Bach S., Baratte B., Ruchaud S., Barbier G., Burgaud G. and Fleury Y. 2017. Bioactive metabolites from the deep subseafloor fungus Oidiodendron griseum UBOCC-A-114129. Mar. Drugs., Apr 7;15(4). pii: E111. doi: 10.3390/md15040111.
43. Corbel C., Sartini S., Levati E., Colas P., Maillet L., Couturier C., Montanini B.* and Bach S.* 2017. Screening for protein-protein interaction inhibitors using a Bioluminescence Resonance Energy Transfer (BRET) based assay in yeast. SLAS Discovery (formerly Journal of Biomolecular Screening), 22(6):751-759, doi:1:2472555216689530.
42. Sklepari M., Lougiakis N., Papastathopoulos A., Pouli N., Marakos P., Myrianthopoulos V., Robert T., Bach S., Mikros E. and Ruchaud S. 2017. Synthesis, docking study and kinase inhibitory activity of a number of new substituted pyrazolo[3,4-c]pyridines. Chem Pharm Bull (Tokyo). 65(1):66-81. doi: 10.1248/cpb.c16-00704.
41. Daniilides K., Lougiakis N., Evangelidis T., Kostakis I.K., Pouli N., Marakos P., Mikros E., Skaltsounis AL., Bach S., Baratte B., Ruchaud S., Karamani V., Papafotika A., Christoforidis S., Argyros O., Kouvari E. and Tamvakopoulos C. 2016. Discovery of new aminosubstituted pyrrolopyrimidines with antiproliferative activity against breast cancer cells and investigation of their effect towards the PI3Kα enzyme. Anticancer Agents Med Chem. 2017 Dec 7. [Epub ahead of print]
40. Ambeu N'ta C., Déliko Dago C-D., Coulibaly W-K., Békro Y-A., Mamyrbekova-Békro JA., Foll-Josselin B., Defontaine A., Delehouzé C., Bach S., Ruchaud S., Le Guével R., Corlu A., Jéhan P., Lambert F., Le Yondre N. and Bazureau J-P. 2016. Microwave synthesis of new 3-(3-aminopropyl)-5-arylidene-2-thioxo-1,3-thiazolidine-4-ones as potential Sr/Thr proteine kinase inhibitors. Medicinal Chemistry Research. 25, accepted for publication. DOI: 10.1007/s00044-016-1719-3.
39. Hedidi M., Erb W., Bentabed-Ababsa G., Chevallier F., Picot L., Thiéry V., Bach S., Ruchaud, S., Roisnel T., Dorcet, V. and Mongin, F. 2016. Synthesis of N-pyridyl azoles using a deprotometalation-iodolysis-N-arylation sequence and evaluation of their antiproliferative activity in melanoma cells. Tetrahedron, 72, 6467-6476. DOI: 10.1016/j.tet.2016.08.056.
38. Lawson M., Rodrigo J., Baratte B., Robert T., Delehouzé C., Lozach O., Ruchaud S., Bach S., Brion J.-D., Alami M., Hamze A. 2016. Synthesis, biological evaluation and molecular modeling studies of Imidazo[1,2-a]pyridines derivatives as protein kinase inhibitors. Eur J Med Chem., 123:105-14. doi: 10.1016/j.ejmech.2016.07.040. Epub 2016 Jul 21.
37. Bazin M.A., Rousseau B., Marhadour S., Tomasoni C., Evenou P., Piessard S., Vaisberg A.J., Ruchaud S., Bach S., Roussakis C., Marchand P. 2016. Discovery of Novel (Imidazo[1,2-a]pyrazin-6-yl)ureas as Antiproliferative Agents Targeting P53 in Non-small Cell Lung Cancer Cell Lines. Anticancer Res., 36(4):1621-1630.
36. Motuhi S-E., Mehiri M., Payri C. E., La Barre S.* and Bach S.* 2016. Marine natural products from New Caledonia – a review. Marine Drugs. 14(3), 58; doi:10.3390/md14030058.
35. Moine E., Dimier-Poisson I., Enguehard-Gueiffier C., Logé C., Pénichon M., Moiré N., Delehouzé C., Foll-Josselin B., Ruchaud S., Bach S., Gueiffier A., Debierre-Grockiego F. and Denevault-Sabourin C. 2015. Development of new highly potent imidazo[1,2-b]pyridazines targeting Toxoplasma gondii calcium-dependent protein kinase 1. Eur J Med Chem. 2015 Oct 9;105:80-105. doi: 10.1016/j.ejmech.2015.10.004.
34. Marquise N., Tai Nguyen T., Chevallier F., Picot L., Thiéry V., Lozach O., Bach S., Ruchaud S., Mongin F. 2015. Azine and Diazine Functionalization using 2,2,6,6-Tetramethylpiperidino-Based Lithium-Metal Combinations. Application to the Synthesis of 5,9-Disubstituted Pyrido[3’,2’:4,5]pyrrolo[1,2-c]pyrimidines. SYNLETT, Georg Thieme Verlag, 2015, 26, pp.2811-2816. <10.1055/s-0035-1560496>.
33. Marchand P., Bazin M.A., Pagniez F., Rivière G., Bodero L., Marhadour S., Nourrisson M-R., Picot C., Ruchaud S., Bach S., Baratte B., Sauvain M., Castillo Pareja D., Vaisberg A., Le Pape P. 2015. Synthesis, antileishmanial activity and cytotoxicity of 2,3-diaryl- and 2,3,8-trisubstituted imidazo[1,2-a]pyrazines. Eur. J. Med. Chem., 103, 381-395. doi: 10.1016/j.ejmech.2015.09.002.
32. Déliko Dago C.D., N'Ta Ambeu C., Coulibaly W.K., Bekro Y.A., Mamyrbekova J., Defontaine A., Baratte B., Bach S., Ruchaud S., Le Guével R., Ravache M., Corlu A., Bazureau J-P. 2015. Synthetic Development of New 3-(4-Arylmethylamino)butyl-5-arylidene-rhodanines Under Microwave Irradiation and their Effects on Tumor Cell Lines and against Protein Kinases. Molecules. 20, 12412-12435; doi:10.3390/molecules200712412.
31. Corbel C., Zhang B., Le Parc A., Baratte B., Colas P., Couturier C., Kosik S. K., Landrieu I., Le Tilly V., and Bach S.* 2015. Tamoxifen Inhibits CDK5 Kinase Activity by Interacting with p35/p25 and Modulates the Pattern of Tau Phosphorylation. Chemistry & Biology 22 (4), 472-482. *corresponding author.
30. Baratte B., Serive B. et Bach S*. 2015. Le criblage à Roscoff: une recherche d'inhibiteurs de kinases tournée vers la mer; [Screening marine resources to find novel chemical inhibitors of disease-relevant protein kinases]. Médecine & Sciences. 31(5); 538-545 *corresponding author.
29. Hochard A., Reininger L., Ruchaud S. and Bach S*. 2014. High-Throughput Screening of marine resources Chapter of the textbook ”Outstanding Marine Molecules and New Trends in Analytical Methods” edited by J-M Kornprobst and S. La Barre, Wiley-VCH, ISBN: 978-3-527-33465-0. *corresponding author.
28. Abida H., Ruchaud S., Rios L., Humeau A., Probert I., De Vargas C., Bach S.*, Bowler C.* 2013. Bio-prospecting the Ocean’s Plankton. Mar. Drugs, 11, 4594-4611; doi:10.3390/md11114594. *corresponding authors.
27. Corbel C., Wang Q., Bousserouel H., Hamdi A., Zhang B., Lozach O., Ferandin Y., Tan V.B., Guéritte F., Colas P., Couturier C. and Bach S. 2011. First BRET-based screening assay performed in budding yeast leads to the discovery of CDK5/p25 interaction inhibitors. Biotechnol J. 6(7):860-870.
26. Zhang B., Corbel C., Guéritte F., Couturier C., Bach S. and Tan V.B. 2011. An in silico approach for the discovery of CDK5/p25 interaction inhibitors. Biotechnol J. 6(7):871-881.
25. Menn B., Bach S., Blevins T.L., Campbell M., Meijer L. and Timsit S. 2010. Delayed treatment with systemic (S)-roscovitine provides neuroprotection and inhibits in vivo CDK5 activity increase in animal stroke models. PLoS One. 5(8):e12117.
24. Gug F., Oumata N., Tribouillard-Tanvier D., Voisset C., Desban N., Bach S., Blondel M. and Galons H. 2010. Synthesis of conjugates of 6-aminophenanthridine and guanabenz, two structurally unrelated prion inhibitors, for the determination of their cellular targets by affinity chromatography. Bioconjug Chem. 21:279-288.
23. Sasvari Z., 1, Bach S., Blondel M. and Nagy P. 2009. Inhibition of RNA recruitment and replication of an RNA virus by acridine derivatives with known anti-prion activities. PLoS One. 4(10):e7376.
22. Corbel C., Haddoub R., Guiffant D., Lozach O., Gueyrard D., Lemoine J., Ratin M., Meijer L., Bach S. and Goekjian P. 2009. Identification of potential cellular targets of aloisine A by affinity chromatography. Bioorgan. Med. Chem. 17(15):5572-82.
21. Tribouillard-Tanvier D., Dos Reis S., Gug F., Voisset C., Béringue V., Sabate R., Kikovska E., Talarek N., Bach S., Huang C., Desban N., Saupe S. J., Supattapone S., Thuret J-Y., Chédin S., Vilette D., Galons H., Sanyal S. and Blondel M. 2008. Protein folding activity of rRNA is a selective target of two unrelated antiprion drugs. PLoS One, 3(5): e2174.
20. Tribouillard-Tanvier D., Béringue V., Desban N., Gug F., Bach S., Voisset C., Galons H., Laude H., Vilette D. and Blondel M. 2008. Antihypertensive drug Guanabenz is active in vivo against both yeast and mammalian prions. PLoS One, 3(4): e1981.
19. Bacart J., Corbel C., Jockers R., Bach S. and Couturier C. 2008. The BRET technology and its application to screening assays. Biotechnology Journal, 3:311-324.
18. Tribouillard D., Gug F., Galons H., Bach S., Saupe S.J. and Blondel M. 2007. Antiprion drugs as chemical tools to uncover mechanisms of prion propagation. Prions, 1:48-52.
17. Guiffant D., Tribouillard D., Gug F., Galons H., Meijer L., Blondel M. and Bach S. 2007. Identification of intracellular targets of small molecular weight chemical compounds using affinity chromatography. Biotechnology Journal, 2:68-75.
16. Iurisci I., Filipski E., Reinhardt J., Bach S., Gianella-Borradori A., Lacobelli S., Meijer L. and Lévi F. 2006. Improved tumor control through circadian clock induction by Seliciclib, a cyclin-dependent kinase inhibitor. Cancer Research 66:10720-10728.
15. Bach S., Blondel M. and Meijer L. 2006. Evaluation of CDK inhibitor selectivity: from affinity chromatography to yeast genetics. In “Monographs on enzyme inhibitors”, Volume 2. CDK inhibitors and their potential as anti-tumor agents » (E. Yue and P.J. Smith, editors). CRC Press, Taylor & Francis, chapter 5, 103- 119.
14. Bach S., Tribouillard D., Talarek N., Desban N. and Blondel M. 2006. A yeast-based assay to isolate drugs active against mammalian prions. Methods 39:72-77.
13. Le Sourd F., Bellé R., Bach S., Boulben S., Cormier P. and Mulner-Lorillon O. 2006. Cellular coexistence of two high molecular subsets of eEF1B complex. FEBS Letters 580:2755-2760.
12. Deschamps C., Bach S., Portetelle D. and Vandenbol M. 2006. The Tre2 oncoprotein, implicated in Ewing’s sarcoma, interacts with two components of the cytoskeleton. Biotechnology letters 28:223-231.
11. Tribouillard D., Bach S., Gug F., Desban N., Beringue V., Andrieu T., Dormont D., Galons H., Laude H., Vilette D. and Blondel M. 2006. Using budding yeast to screen for anti-prion drugs. Biotechnology Journal 1: 58-67.
10. Tang L., Li MH., Cao P., Wang F., Chang WR., Bach S., Reinhardt J., Ferandin Y., Galons H., Wan Y., Gray N., Meijer L., Jiang T. and Liang DC. 2005. Crystal structure of pyridoxal kinase in complex with roscovitine and derivatives. J. Biol. Chem. 280: 31220-31229.
9. Bach S., Knockaert M., Reinhardt J., Lozach O., Schmitt S., Baratte B., Koken M., Coburn S., Tang L., Jiang T., Liang D-C., Galons H., Dierick J-F., Pinna L., Meggio F., Totzke F., Schächtele C., Lerman A., Carnero A., Wan Y., Gray N. and Meijer L. 2005. Roscovitine targets: protein kinases and pyridoxal kinase. J. Biol. Chem. 280: 31208-31219.
8. Blondel M., Bach S., Bamps S., Dobbelaere J., Wiget P., Longaretti C., Barral Y., Meijer L. and Peter M. 2005. Degradation of Hof1 by SCFGrr1 is important for actomyosin contraction during cytokinesis in yeast. EMBO Journal 24: 1440-1452.
7. Adolph S., Bach S., Blondel M., Cueff A., Moreau M., Pohnert G., Poulet S.A., Wichard T. and Zuccharo A. 2004. Cytotoxicity of diatom-derived oxylipins in organisms belonging to different phyla. J. Exp. Biol. 207:2935-2946.
6. Gug F., Bach S., Blondel M., Vierfond J-M., Martin A-S. and Galons H. 2004. A single step synthesis of 6-aminophenanthridines from anilines and 2-chlorobenzonitriles. Tetrahedron 60:4705-4708.
5. Knockaert M., Blondel M., Bach S., Leost M., Elbi C., Hager G., Nagy S., Han D., Denison M., Ffrench M., Ryan X.P., Magiatis P., Polychronopoulos P., Greengard P. Skaltsounis L. and Meijer L. 2004. Independent actions on cyclin-dependent kinases and aryl hydrocarbon receptor mediate the anti-proliferative effects of indirubins. Oncogene 23:4400-4412.
4. Wan Y., Hur W., Cho C.Y., Liu Y., Adrian F.J., Lozach O., Bach S., Mayer T., Fabbro D., Meijer L. and Gray N.S. 2004. Synthesis and target identification of hymenialdisine analogs. Chemistry and Biology 11:1-13.
3. Bach S., Talarek N., Andrieu T., Vierfond J-M., Mettey Y., Galons H., Dormont D., Meijer L., Cullin C. and Blondel M. 2003. Isolation of drugs active against mammalian prions using a yeast-based screening assay. Nature Biotechnology 21:1075-1081. Article commented in : Saupe S. J. 2003. New anti-prion drugs make yeast blush. Trends in Biotechnology. 21:516-519.
2. Bizimungu C., De Neve N., Burny A., Bach S., Bontemps F., Portetelle D. and Vandenbol M. 2003. Expression in a RabGAP yeast mutant of two human homologues, one of which is an oncogene. Biochem. Biophys. Res. Commun. 310:498-504.
1. Bach S., Bouchat O., Portetelle D. and Vandenbol M. 2000. Co-deletion of the yeast MSB3 and MSB4 coding regions affects bipolar budding and perturbs the organisation of the actin cytoskeleton. Yeast 16:1015-1023.
